Home
/
medtech
/
in vitro diagnostics
CAPABILITIES
WHAT WE DO
We can support your business with:
- Engineering services covering all stages of the Product Development lifecycle, from Feasibility Analysis (ValueSPRINT™) to Product Verification
- EMC & Electrical Safety Product Certification at our in-house accredited facilities
- Product Industrialization and Manufacturing, Supply & On-market Support
- ISO 13485 Certified for IVD Development & Manufacturing, including compliance to IEC 60601

TECHNOLOGIES
How we do it
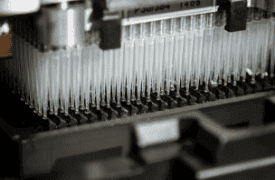
- End-to-end automation of Test Methods, including
Sample and Reagent Management, biochemistry test integration, detection and test result post-processing - Development of customized solutions based on the use of standard Fluid Handling platforms
- Artificial Intelligence-based pre-analytical and
post-analytical solutions - Instrument Firmware and Software
- Connectivity and Cybersecurity
- Design for Manufacturing